Glucose: Definition, Functions, and Cellular Reactions
1. What is Glucose?
Glucose is a simple sugar (monosaccharide) that serves as the primary energy source for most living organisms. Its molecular formula, C₆H₁₂O₆, represents its composition of carbon, hydrogen, and oxygen atoms. Found naturally in foods and produced by plants during photosynthesis, glucose is crucial for energy production in both plants and animals.
In humans, glucose is a central player in metabolism, providing fuel for cells through a series of biochemical reactions. Its regulation in the bloodstream is vital for maintaining energy balance and overall health.
---
2. Sources of Glucose
The primary sources of glucose include:
Carbohydrate-rich foods:
Fruits: Grapes, bananas, and apples.
Vegetables: Potatoes, corn, and carrots.
Grains: Rice, wheat, and oats.
Processed foods: Sugary snacks, soft drinks, and glucose syrups.
Body’s internal processes: The liver can produce glucose from non-carbohydrate sources (gluconeogenesis) when dietary intake is insufficient.
---
3. Role of Glucose in the Body
Glucose is indispensable for several physiological functions:
Energy Source: It is the body’s primary fuel, especially for high-energy-demand organs like the brain and muscles.
Energy Storage: Excess glucose is stored as glycogen in the liver and muscles, or converted into fat for long-term storage.
Cellular Metabolism: It powers cellular processes through aerobic and anaerobic respiration.
---
4. How Glucose Enters Cells
Glucose uptake into cells is mediated by specific transport mechanisms:
1. Transport through Glucose Transporters (GLUTs): Proteins embedded in cell membranes that facilitate glucose movement.
2. Insulin Role: In insulin-sensitive tissues (like muscles and fat), insulin binds to receptors, triggering GLUT4 transporters to move to the cell membrane and allow glucose entry.
Once inside the cell, glucose undergoes complex metabolic reactions to produce energy in the form of adenosine triphosphate (ATP).
---
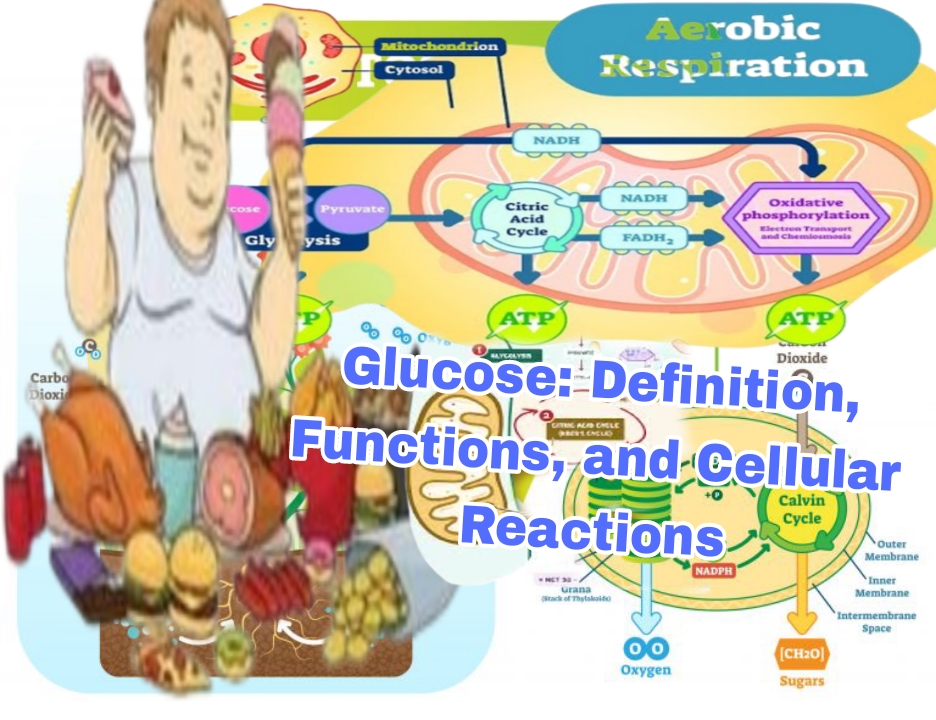
5. Scientific Reactions of Glucose Inside Cells
5.1 Glycolysis
Location: Cytoplasm of the cell.
Function: Converts glucose into pyruvate, generating energy intermediates.
Steps:
1. Glucose is phosphorylated to glucose-6-phosphate using ATP and the enzyme hexokinase.
2. Glucose-6-phosphate is converted to fructose-6-phosphate, then to fructose-1,6-bisphosphate.
3. Fructose-1,6-bisphosphate is split into two molecules: glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP).
4. G3P undergoes oxidation, producing NADH and ATP.
5. The final product is two molecules of pyruvate, along with a net gain of 2 ATP and 2 NADH.
Key Features:
Occurs without oxygen (anaerobic).
Provides a quick energy source for cells.
---
5.2 Pyruvate Oxidation
Location: Mitochondrial matrix.
Process:
1. Pyruvate enters the mitochondria and is converted into acetyl-CoA by the enzyme pyruvate dehydrogenase.
2. This step releases carbon dioxide and produces NADH.
Significance: Acetyl-CoA is the entry molecule for the Krebs cycle.
---
5.3 Krebs Cycle (Citric Acid Cycle)
Location: Mitochondrial matrix.
Function: Oxidizes acetyl-CoA to produce electron carriers.
Steps:
1. Acetyl-CoA combines with oxaloacetate to form citrate.
2. Through a series of enzymatic reactions, citrate is oxidized, releasing 2 CO₂ molecules.
3. The cycle produces 3 NADH, 1 FADH₂, and 1 GTP (or ATP) per turn.
Key Features:
Central hub for metabolism, connecting carbohydrates, fats, and proteins.
Generates intermediates used in biosynthesis.
---
5.4 Electron Transport Chain (ETC) and Oxidative Phosphorylation
Location: Inner mitochondrial membrane.
Process:
1. NADH and FADH₂ donate electrons to the ETC.
2. Electrons are passed through protein complexes, creating a proton gradient across the membrane.
3. Protons flow back into the mitochondrial matrix through ATP synthase, driving the synthesis of ATP.
Yield: Approximately 32-34 ATP molecules per glucose molecule.
Significance: ETC is the final stage of glucose oxidation, producing the majority of cellular ATP.
---
6. Anaerobic Respiration
When oxygen is limited, cells rely on anaerobic pathways:
Lactic Acid Fermentation: Pyruvate is converted to lactate, regenerating NAD⁺ for glycolysis to continue.
Yield: 2 ATP per glucose molecule.
Anaerobic respiration is less efficient but critical during high-intensity activities or in oxygen-deprived conditions.
---
7. Glucose Regulation in the Body
Maintaining stable blood glucose levels is crucial for health. Key regulatory mechanisms include:
Insulin: Lowers blood glucose by promoting cellular uptake and storage as glycogen.
Glucagon: Raises blood glucose by stimulating glycogen breakdown in the liver.
---
8. Disorders Related to Glucose
Diabetes Mellitus:
Type 1: Autoimmune destruction of insulin-producing cells.
Type 2: Insulin resistance in cells.
Hypoglycemia: Low blood glucose, leading to fatigue, dizziness, and confusion.
Hyperglycemia: High blood glucose, potentially damaging organs over time.
---
9. Applications of Glucose in Science and Industry
Medical Use: Glucose is a critical component in IV fluids for energy support in patients.
Biotechnology: Used in fermentation processes to produce ethanol, antibiotics, and enzymes.
Food Industry: Acts as a natural sweetener and a substrate for caramelization.
---
10. Maintaining Healthy Glucose Levels
Balanced Diet: Include complex carbohydrates like whole grains, fruits, and vegetables.
Regular Exercise: Improves insulin sensitivity and glucose uptake by muscles.
Weight Management: Helps prevent insulin resistance.
---
Conclusion
Glucose is a cornerstone of cellular metabolism and energy production. Through its intricate biochemical pathways, it supports the body's energy demands and sustains vital functions. Understanding glucose’s role and the processes it undergoes within cells highlights its significance in health, medicine, and industry. Maintaining a balanced glucose level is essential for overall well-being, underscoring the importance of a healthy lifestyle and diet.

إرسال تعليق